Physicist David Altman is moved by myosin. We all are.
Myosin is a motor protein. Responsible for every conscious and unconscious flex of our muscles, it beats our hearts.
Altman wants to understand how the molecular motor works — how the roiling action inside of a cell affects myosin's function. In 2012, Altman spent his junior sabbatical at McGill University in Montreal, Canada, performing an experiment that tested competing theories about muscle fibers’ unusual mechanical properties. The results of his experiment were published April 16 in PLoS ONE.
Muscles and Motors
Muscles have an odd and poorly understand behavior. Normally, an active muscle is stiff, because its proteins interact and form connections. But when an active muscle is used in a cyclic fashion — think of your heart beating or leg muscles when you’re running — the muscle temporarily becomes softer. When the cyclic motion stops, the muscle stiffens again.
This temporary softening, called thixotropy, may be connected to our posture. Stiff muscles provide support when you sit in class, but become less stiff and more limber when you run. The mechanism of thixotropy in muscles is not well understood, though Altman suspected that myosin was the key.
At the heart of muscle fibers are two types of interlocking filaments: myosin filaments — which contain hundreds of myosin motors that tie themselves together — and actin filaments. In active muscle, myosins bind to actin. Myosins make muscles contract by pulling themselves, stroke by stroke, along the actin filament.
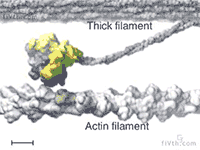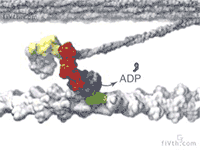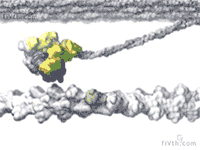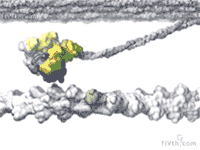
Loosening Up
Altman wanted to test whether the interaction between actin and myosin was responsible for muscle’s thixotropy. According to this model, muscles’ cyclic motion breaks the actin-myosin attachments and makes the muscle fiber softer. Once motion stops, it takes a little while for myosin to re-bind to actin and make the fiber stiff again.
Altman’s experiment involved disrupting actin-myosin interactions and vibrating the muscle fiber at different frequencies to measure its stiffness. Using two chemicals that prevent myosin binding to actin, Altman then tested the muscle. The loosening effect vanished.
“To people who study muscle, it was a debate as to what was responsible for thixotropy,” says Altman. ”The experiment indicates that muscles’ stiffness depends on myosin’s grip.”
Next Steps
The experiment revealed another, unknown process at work. When the cyclic motion was slow enough, Altman was surprised to find that the muscle fiber no longer softened; it became stiffer, or rheopectic. Altman suspects thixotropy depends on forces from molecules jostling within the crowded cellular environment, but no one knows how or why muscles would stiffen when subjected to low frequency vibration.
Through Willamette’s Senior Research Seminar course and through the Science Collaborative Research Program, students work with Altman to learn more about myosins’ cellular function. “In my lab, we study myosins inside cells as opposed to just purified myosins, because we want to understand physiologically relevant conditions and how they affect the motor,” says Altman.
Over the summer, he’ll move his myosin-trapping laser lab to the basement of Collins Science Center. In the fall, Altman will continue working with physics majors on their theses, while looking for more collaborative research opportunities to explore the enigmatic muscular motor protein.

